“Do you ever think that your hair loss, dull skin, sore throat, dry mouth, allergies or other health problems could have some relation with water quality?” Yijing Stehle asked. “I am inspired by the question, ‘How safe is our water?’”
To find out, she’s studying a group of chemical contaminants called volatile organic compounds (VOCs).
“VOCs come not only from big manufacturing companies like GE, IBM and GlobalFoundries, but from almost all businesses and even households,” Stehle said. “VOCs are used in paint, solvents, aerosol sprays, cleaners, disinfectants, automotive products and dry cleaning fluids.”
“Even our daily facial products contain VOCs – like benzene in sunscreen,” she added. “And every time you print something, that ink is typically about 10% or 20% VOCs, in the form of petroleum distillates.”
As a result, volatile organic compounds are everywhere in the environment – from the air we breathe to the water we swim in and even drink. Dichloromethane, ethylene glycol and benzene are most common in waterways in upstate New York.
Their ubiquity makes knowing more important.
“Repeated and direct exposure to VOCs can cause serious health effects, including damage to the liver, kidneys and central nervous system,” Stehle said.
And that’s why she’s working to develop better sensors to detect these chemicals.
Sensors currently on the market are mainly working to detect VOCs in the air, but they are expensive and complicated to make, and they aren’t stable under ambient conditions for long-term storage or use.
But making a better sensor is tricky – sensitivity and compatibility are often hard to combine.
Partly because volatile organic compounds are, well, volatile. They can easily volatilize (evaporate) into the air or dissolve in water, which means a good sensor has to be able to handle both scenarios.
“When it comes to VOCs, drinking is no better than inhaling,” Stehle said. “So we want to make our sensor good for both dry and wet environments.”
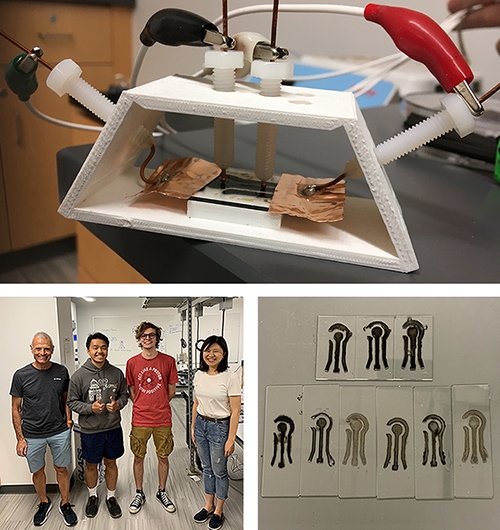
She and her research team are focusing their efforts on printable tungsten disulfide sensors. The sensors are made by dropping layers of graphene ink onto a mold, which is then placed in a humidity chamber and hooked up to a set of electrodes.
“Data will be taken for VOCs of different concentrations to confirm the response and sensitivity of the sensor. When water is used, it will be taken from local waterways,” Stehle said. “The final cost for each sensor should be less than 5 cents, which makes it disposable.”
The team includes mechanical engineering technology coordinator Stan Gorski, engineering lab technician Robert Harlan, Zoe Lyon ’22, Hayden Qualls ’22, Sang Duong ’23 and Luke Kilby ’23.
Their work is supported by a grant from the Bender Scientific Fund of the Community Foundation for the Greater Capital Region.